Dedicated to developing
life-changing medicines
for patients and families living with challenging diseases

Dedicated to developing
life-changing medicines
for patients and families living with challenging diseases

Driven by the desire to deliver better therapeutic options for patients in need

Driven by the desire to deliver better therapeutic options for patients in need

Driven by the desire to deliver better therapeutic options for patients in need
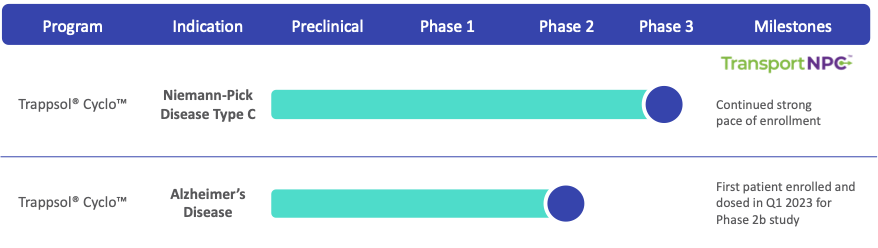
Virtual Investor: Alzheimer’s Disease Spotlight
Virtual Investor: Niemann-Pick Disease Type C Spotlight